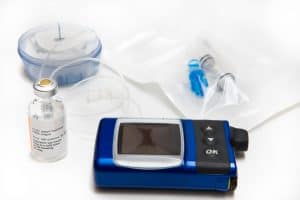
Medtronic Insulin Pump Recalled.
Medtronic has recalled some of their insulin pumps after one death and several injuries were reported.
The recall includes certain MiniMed 600 Series pumps for delivering incorrect dosages of insulin to patients.
These devices help those with diabetes manage correct blood glucose levels by delivering insulin to the body.
According to Medtronic, the recalled pumps have a missing or broken retainer ring, which helps lock the insulin cartridge in place in the pump’s reservoir. Due to this, patients can receive too much or too little insulin.
The FDA has received 26,421 complaints about the device malfunctioning: including 2,175 injuries and one death.
The recall includes about 322,000 pumps. The names of the recalled pumps are below:
- Model 630G disbursed to patients in September 2016 to October 2019
- Model 670G disbursed to patients in June 2017 to August 2019
The FDA says patients who use the recalled pumps should consult their doctors. If you have questions about the recall, you can call the 24-hour Medtronic Technical Support line at 877-585-0166.
Recovering compensation after an injury from a defective product
It is a manufacturer’s responsibility to make sure their products are safe for consumers. If someone is injured due to a defective product, a product liability claim may exist. A claim may be pursued to pay for damages caused by the defective product such as medical bills, missed time from work and pain and suffering.
40+ years of experience from strong, knowledgeable, compassionate attorneys.
Start A Free EvaluationIf you or someone you know has been seriously injured by a defective product, it is important to contact a personal injury attorney to review your rights. Call 412-661-1400 to schedule a free consultation with a personal injury lawyer in Pittsburgh at Berger and Green today.
Source: CNN Health, “Medtronic recalls certain MiniMed insulin pumps tied to 1 death”







